Evidence-Based Practice Library Guide
In This Guide
What is Evidence-Based Practice?
Forming a Clinical Question (PICO)
Inclusion & Exclusion Criteria
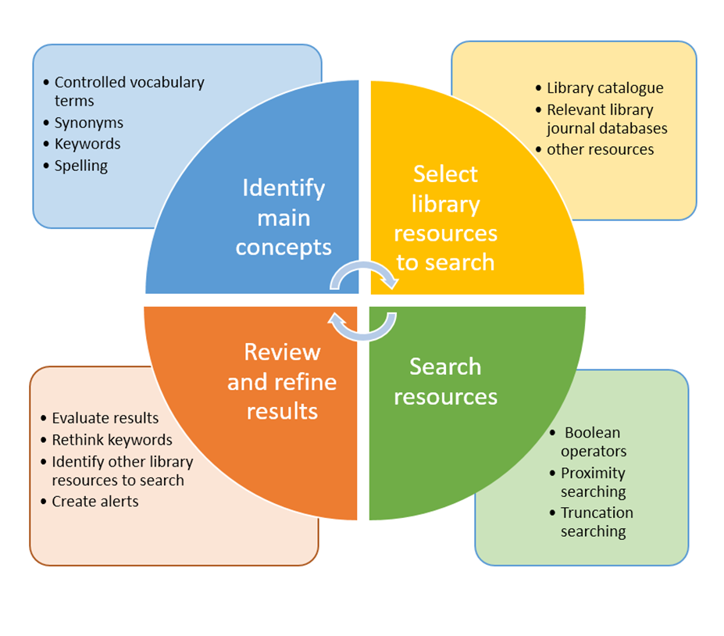
Acquiring Evidence
Appraising the Quality of Evidence
Writing a Literature Review
Keeping Track of Your Sources
Evidence-Based Practice Steps
Ask a question about patient care. Examples: Prognosis, harm, or diagnosis.
Clarify your question using PICO:
Patient/Population or Problem
Intervention
Comparison
Outcomes
Search for an existing answer in research.
Evaluate the answer you find. Does it fit with your patient’s values and with the resources you have on hand?
Carry out the process.
What is Evidence-Based Practice?
Evidence-based practice is a method of using critically appraised and scientifically proven evidence to deliver quality health care to a specific population.
Evidence-based practice involves answering clinical questions using a combination of information from existing research, your clinical expertise, your patient's values, and local resources.
To identify the best research evidence, you must:
Create your clinical question (utilizing PICO).
Search high quality resources to acquire the best information to address your question.
Appraise and apply the evidence collected.
Forming a Clinical (PICO) Question
Each discrete topic: your P, your I, your C and your O, will likely all have different ways of being expressed when different authors write about them, so when breaking down your question into concepts (search terms), think of and use synonyms.
When you use databases or search engines, they retrieve what you type in so it is up to you to think of how an author might express your topics in their writing in order to expand the possibility of catching all the relevant articles.
"Leadership: Ask Better Questions" by Joel Mark Witt is licensed under CC BY-NC-ND 2.0.
A specific nursing and health sciences search mechanism is something called “PICO Searching.”
This mnemonic device not only helps you create a useful research question, but also helps you create a search string to use in databases.
P stands for…Population/Patient/Problem:
What is the problem to be addressed or the population involved?
I stands for…Intervention:
What intervention or factor or is being tested/considered?
C stands for…Comparison:
Is there an alternative to compare with the intervention? (Sometimes there is not.)
O stands for… Outcome:
What can be measured?
This is a set of criteria that you determine when searching for literature. They set boundaries for what results you’ll get when searching.
The most straightforward way to do this is to use filters in databases. (After determining your criteria.)
Inclusion Criteria are the elements of an article that must be present for it to be eligible for inclusion in a literature review.
Examples could include:
Studies of a certain type (e.g., only Randomized Controlled Trials)
Studies conducted in certain geographic area (U.S., Canada, etc.)
Studies published in the last 5 years.
Exclusion Criteria are the elements of an article that disqualify a study from inclusion in a literature review.
Examples could include:
Study used qualitative methodology
Study was published more than 5 years ago
Study was published in a language other than English
Inclusion and Exclusion Criteria
Acquiring Evidence
Types of Evidence:
The type of question you need to answer helps you determine what type of study to look for.
All Clinical Questions:
Systematic review: A comprehensive summary of high-quality studies examining a given topic.
Meta-analysis: A type of systematic review in which results from available high-quality studies are statistically combined to compute a net overall effect
Clinical practice guideline: A statements that includes recommendations intended to optimize patient care. Guidelines are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options.
Therapy:
Randomized controlled trial: A carefully planned type of epidemiologic study in which participants are randomly assigned to receive a given exposure (such as a new drug or therapy) and then followed to examine the effects of the exposure on outcomes.
Cohort study: A type of epidemiologic study design where one or more population groups (called cohorts) are classified according to their level of exposure to a given agent/risk factor and followed over time to determine if this exposure is related to the occurrence of a disease or outcome of interest.
Case-control study: A type of epidemiologic study that compares individuals who have a disease or outcome of interest (cases) with those who do not (controls).
Case series: A summary of a small group of individuals’ experience with a similar disease or outcome of interest.
Etiology/Harm:
Randomized controlled trial: A carefully planned type of epidemiologic study in which participants are randomly assigned to receive a given exposure (such as a new drug or therapy) and then followed to examine the effects of the exposure on outcomes.
Cohort study: A type of epidemiologic study design where one or more population groups (called cohorts) are classified according to their level of exposure to a given agent/risk factor and followed over time to determine if this exposure is related to the occurrence of a disease or outcome of interest.
Case-control study: A type of epidemiologic study that compares individuals who have a disease or outcome of interest (cases) with those who do not (controls).
Case series: A summary of a small group of individuals’ experience with a similar disease or outcome of interest.
Diagnosis:
Prospective, blind comparison to a gold standard
(a type of controlled trial)Cohort study: A type of epidemiologic study design where one or more population groups (called cohorts) are classified according to their level of exposure to a given agent/risk factor and followed over time to determine if this exposure is related to the occurrence of a disease or outcome of interest.
Cross-sectional study: A type of epidemiologic study that observes the relationship between a characteristic/risk factor (the exposure) and the prevalence of the disease or outcome of interest in a specific population at a single point in time.
Prognosis:
Cohort study: A type of epidemiologic study design where one or more population groups (called cohorts) are classified according to their level of exposure to a given agent/risk factor and followed over time to determine if this exposure is related to the occurrence of a disease or outcome of interest.
Case-control study: A type of epidemiologic study that compares individuals who have a disease or outcome of interest (cases) with those who do not (controls).
Case series: A summary of a small group of individuals’ experience with a similar disease or outcome of interest.
Prevention:
Randomized controlled trial: A carefully planned type of epidemiologic study in which participants are randomly assigned to receive a given exposure (such as a new drug or therapy) and then followed to examine the effects of the exposure on outcomes.
Cohort study: A type of epidemiologic study design where one or more population groups (called cohorts) are classified according to their level of exposure to a given agent/risk factor and followed over time to determine if this exposure is related to the occurrence of a disease or outcome of interest.
Case-control study: A type of epidemiologic study that compares individuals who have a disease or outcome of interest (cases) with those who do not (controls).
Case series: A summary of a small group of individuals’ experience with a similar disease or outcome of interest.
Where to Search
Content type: articles, books, reference info, and video. Provides full-text access to over 600 journals, 1,000 books, and 9,000 medical and procedural videos.
Content types: articles & books. Provides full text of more than 300 nursing and allied health journals and indexing of more than 5,500.
Content type: reference info. Clinical reference tool that assists with point-of-care needs.
Dynamic Health CDS + Skills
Content types: reference info, video, and images. Evidence-based information resource containing disease, conditions, and drug topics as well as videos and images.
Content type: articles. PubMed contains more than 30 million citations and abstracts of biomedical literature.
Unable to find evidence?
Sometimes, despite your best efforts, you find few or no articles related to your clinical question. If you are unable to find evidence, here are some strategies to try:
Do some preliminary research to see what you can find, and design your PICO around the available evidence. In other words, find an alternative assessment, treatment, or service delivery option that is evidence-based.
Reconsider your search terms. Try adding more synonyms to your search terms.
Consider research from similar or related populations, interventions, or outcomes. Use your best judgment to decide if such information could be helpful for your paper.
Still struggling? Contact a librarian!
Librarian for Health Sciences:
Emma Fernandez (she/her)
efernandez@stephens.edu
(573) 441-5129
Appraising the Quality of Evidence
Critical Appraisal: “The process of systematically examining research evidence to judge its trustworthiness, its value, and relevance in a particular context."
Some questions to consider when looking at articles:
Does this study address a clearly focused question?
Did the study use valid methods to address this question?
Are the valid results of this study important?
Are these valid, important results applicable to my patient or population?
If the answer to any of these questions is “no”, you can save yourself the trouble of reading the rest of it.
Writing a Literature Review
A literature review is an analysis of scholarly writings that relate to your research question. It helps provides background information on your topic and shows a connection between the studies you find and your research question.
When searching for the evidence or working under the title of 'evidence based' it's best to be as detailed and thorough as possible.
This means:
documenting your work - where & how you searched
searching multiple databases;
using multiple terms for each of your PICO concepts
using AND/OR/NOT to search
Keeping Track of Your Sources Using Zotero
Zotero is a free citation management program that assists with collecting, organizing, citing, and sharing research. It allows you to:
Compile and organize all your sources in one place
Annotate and create notes on sources
Access your sources from different locations (on a desktop or on the web)
Read and share PDFs
Format references to create in-text citations and bibliographies
In order to use Zotero, first download the Zotero software to your computer and install the Zotero Connector to your internet browser.
It is also beneficial to register for a free Zotero account. Having an account will allow you to sync and access your library from anywhere. It will also allow you to join groups and back up any attached files.
Check out our Zotero Library Guide.